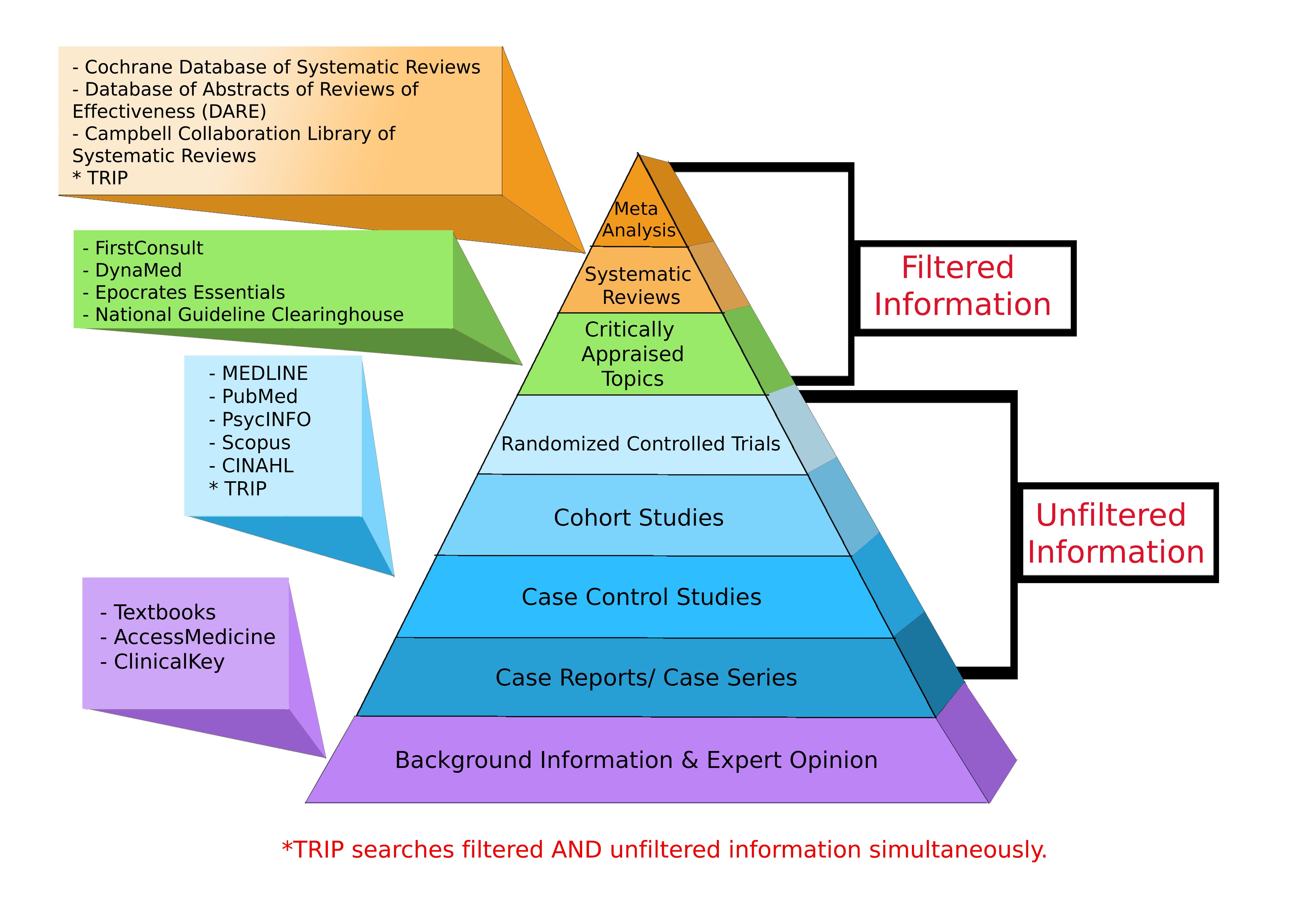
The KansasCOM Library provides access to most of the resources identified on the Levels of Evidence Pyramid. The links below will take you directly to those resources.
Meta-Analysis & Systematic Reviews
Critically Appraised Topics
Randomized Controlled Studies, Cohort Studies, Case Control Studies, Case Reports/Case Series
Background Information & Expert Opinions
ACM provides residents, nurse practitioners, and physician assistants with interactive content, self-assessment, and leading medical texts to enhance decision-making at the point-of-care. It and allows for practicing physicians to brush up on their medical knowledge to ensure best patient outcomes. It includes a complete full-text library of clinical and basic science textbooks, including Harrison’s Principles of Internal Medicine, 20e, CURRENT Medical Diagnosis & Treatment, The Color Atlas of Family Medicine, 2e, and many others. The database is indexed in One Search.
An observational study is a study in which the investigator cannot control the assignment of treatment to subjects because the participants or conditions are not directly assigned by the researcher.
In an experimental study, the investigators directly manipulate or assign participants to different interventions or environments
Experimental studies that involve humans are called clinical trials. They fall into two categories: those with controls, and those without controls.
Definitions taken from: White SE. Basic & Clinical Biostatistics. 5th ed. New York, N.Y: McGraw-Hill Education LLC; 2020.Retrieved April 21, 2022 from AccessMedicine
Levels of Evidence Pyramid created by Andy Puro, September 2014
Not all resources are available at the KansasCOM Library. Check with Library staff for assistance or visit the full database list.